
Helicobacter pylori (H pylori) infection is among the most common bacterial infections worldwide with approximately one-half of the world’s population infected. H pylori is a Gram-negative, curved bacterial rod, which has been associated with symptoms ranging from those of peptic ulcer and dyspepsia to gastric adenocarcinoma and mucosa-associated lymphoid tissue (MALT) lymphoma. Though gastric adenocarcinoma and MALT lymphoma occur in <1% of infected individuals, peptic ulcer disease and dyspepsia are common and, therefore, accurate diagnosis and prompt initiation of antibiotic therapy are important for successful disease resolution.
Several invasive and noninvasive testing methods are available to detect H pylori infection. The noninvasive methods include serology, the urea breath test (UBT) and the H pylori stool antigen test (SAT). Among these, serologic evaluation for antibodies remains the most commonly ordered assay, despite established guidelines from both the American Gastroenterology Association (AGA) and the American College of Gastroenterologists (ACG) that recommend either the UBT or the SAT as the preferred testing modalities for active H pylori infection.1-3 Though serologic assays are still discussed in the ACG and AGA guidelines, they indicate that due to the persistent nature of antibodies to H pylori, active disease requires confirmatory testing by an alternative assay; eg, UBT or SAT. Notably, serologic testing was omitted as an acceptable diagnostic method for diagnosis of functional dyspepsia in a recent New England Journal of Medicine review article on functional dyspepsia4 by Dr. Nicholas Talley, an expert in the field of gastroenterology and H pylori infection. Finally, an increasing number of health insurance providers, including Aetna, Geisinger Health Plan, and Cigna, are no longer reimbursing patients for H pylori serologic testing.
In this article, we will review the AGA and ACG recommendations, comment on updated practices at Mayo Clinic, and provide clinicians with alternative testing strategies to identify active infection with H pylori.
The diagnostic assays for detection of H pylori infection can be classified as either invasive, requiring gastric endoscopy and tissue biopsy, or noninvasive, requiring readily available specimen types (ie, serum, breath, and stool samples). The specific indications for endoscopy will not be discussed in this article, although, in general, clinicians may wish to rule out malignancies or other noninfectious causes of the patient’s symptoms. When symptoms are considered typical of H pylori disease, noninvasive testing for that infection will be initially performed. The advantages and limitations of the various testing methodologies will be reviewed within this article.
Invasive Tests
Following recovery of 1 or more gastric tissue biopsies, evaluation for the presence of H pylori can be accomplished by 1 of 4 methods—culture, rapid urease tests, histopathology, or molecular testing (See Table).
| Advantages | Limitations | |
|---|---|---|
| Invasive | ||
| Culture | -Evidence of active infection -Antimicrobial susceptibility testing possible -High specificity |
-Sensitivity affected by: Site of biopsy and bacterial load Viability of organism during transport -Not routinely available |
| Rapid Urease Test (RUT) | -Evidence of active infection -Rapid -High sensitivity and specificity (>90%) |
-Sensitivity affected by: Site of biopsy and bacterial load Viability of organisms prior to testing Prior use of PPIs, bismuth, antibiotics - Specificity may be affected by presence of urease from other Helicobacter species |
| Histopathology | -Evidence of active infection | -Sensitivity affected by site of biopsy - Specificity affected by presence of nonpathogenic, curved, gram-negative rods in gastric lining |
| Molecular (RT-PCR) | -High sensitivity and specificity | -Not routinely available |
| Noninvasive | ||
| Urea Breath Test (UBT) | -Evidence of active infection -High sensitivity and specificity -FDA cleared for use in adults -Used to monitor response to therapy -Recommended by AGA and ACG guidelines |
-Specialized specimen collection and processing requirements -Not FDA cleared for use in <18-year-olds -Prior (≤2 weeks) PPI, bismuth or antibiotic use decreases sensitivity -Specificity may be affected by presence of urease from other Helicobacter species |
| Stool Antigen Test (SAT) | -Evidence of active infection -High sensitivity and specificity -FDA cleared for use in children and adults -Used to monitor response to therapy -Ease of specimen collection -Recommended by AGA and ACG guidelines |
-Prior (≤2 weeks) PPI, bismuth or antibiotic use decreases sensitivity -Patient discomfort regarding specimen submission |
| Antibody Detection | -Performance not affected by use of PPIs, bismuth or antibiotics -Routine specimen collection -Monitor H pylori epidemiology |
-Poor PPV and specificity for active H pylori infection -Does not differentiate past from current infection -Cannot be used to monitor response to therapy -Not recommended by AGA or ACG guidelines -An increasing number of insurance providers are no longer reimbursing patients for testing |
Table. Advantages and Limitations of Invasive and Noninvasive Assays for Diagnosis of Helicobacter pylori Infection
Abbreviations:
PPIs, proton pump inhibitors; AGA, American Gastroenterology Association; ACG, American College of Gastroenterology; PPV, positive predictive value
a Mayo Medical Laboratories will discontinue all H pylori serologic testing in February 2016.
Culture
Isolation of H pylori by culture of a biopsy specimen is definitive evidence of active infection and isolates can subsequently be tested for susceptibility to various antimicrobial agents (Figure 1). However, the sensitivity of this method is limited by a number of factors, including: 1) the site of biopsy and the quantity of viable organisms, 2) ability to maintain viability of this fastidious bacterium during transport to the laboratory (<48 hours from specimen collection to culture), and 3) unique culture conditions that are not routinely available in all clinical laboratories.

Rapid Urease Tests
Rapid urease tests (RUTs) are based on a pH color indicator system and are designed to detect the presence of urease, an enzyme produced by H pylori that metabolizes urea to ammonia and carbon dioxide. For this assay, biopsy samples are submerged into the RUT solution, and if ammonia is produced, the pH will increase, leading to a color change that indirectly indicates the presence of H pylori. While these assays perform well (>90% sensitivity and specificity for active infection), the sensitivity decreases by approximately 25% among patients using bismuth-containing compounds, proton pump inhibitors (PPIs), or antibiotics.5 Similar to culture, these assays are limited by the site of biopsy and the ability to maintain viability during specimen transport prior to RUT testing. Additionally, other urease-producing organisms have been identified in the gastric mucosa that may lead to nonspecific RUT results.6
Histopathology
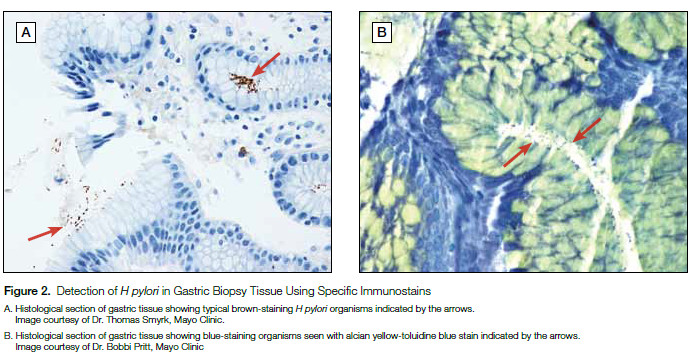
Similar to culture, the detection of H pylori in gastric biopsies by histopathologic evaluation is considered diagnostic for active infection (Figure 2). However, the sensitivity of this method is likewise limited by the site of biopsy and there is a lack of specificity as other nonpathogenic, curved bacteria may reside in the gastric mucosa. Immunohistochemical stains have been developed to improve specificity, though cross-reactivity has been seen with other Helicobacter species (ie, Helicobacter heilmannii).
Real-Time Polymerase Chain Reaction
Finally, molecular evaluation of biopsy specimens for the presence of H pylori DNA by real-time polymerase chain reaction (RT-PCR) is promising and numerous studies have found increased sensitivity and specificity of this method compared to culture, RUT, and histopathology. However, though Mayo Medical Laboratories is working on developing a RT-PCR assay for H pylori from tissue biopsy material, such assays are not widely available in the clinical setting and are currently restricted to research laboratories.
Noninvasive Tests
Three noninvasive assays are available to detect H pylori infection, including 1) urea breath test (UBT), 2) H pylori stool antigen test (SAT), and 3) serologic assays to measure anti-H pylori IgM, IgA, and IgG antibodies.
Urea Breath Test
Similar to the RUT, the principle behind the urea breath test (UBT) is based on production of urease by H pylori and a positive result is indicative of active infection. For the UBT, the patient is asked to ingest a preparation containing 13C-labeled urea—a nonradioactive carbon isotope. If H pylori urease is present in the stomach, the urea is degraded to ammonia and labeled carbon dioxide (13CO2). The labeled 13CO2 is exhaled through normal respiration and collected in a specialized bag. The sample is subsequently analyzed on a spectrophotometer, which determines the level of 13CO2 present. This value is compared to a breath sample taken prior to ingestion of the labeled urea and a final result of positive or negative is determined (Figure 3).

The clinical sensitivity and specificity of the UBT to detect active H pylori infection exceeds 93% in most studies, though a few reports indicate potential cross-reactivity with other gastric urease-producing organisms.6-9 A distinct advantage of this assay is that it can also be used to determine treatment efficacy. Current AGA and ACG guidelines recommend that providers wait at least 4 weeks following cessation of therapy to test for eradication of H pylori by the UBT.10
Despite the excellent performance characteristics, the utility of the UBT is limited by a number of factors. Most notable is the decreased sensitivity of this assay in patients who have taken proton pump inhibitors (PPIs), bismuth-containing products, or antibiotics within 14 days prior to testing. Therefore, it is recommended that these compounds be withheld for 7 to 14 days prior to performing the UBT, if possible.3 Additionally, the UBT assay currently offered through Mayo Medical Laboratories is not approved for use in patients <3 years of age. For individuals <3 years, Mayo Medical Laboratories recommends that the stool antigen test (SAT) be offered instead. Finally, the performance of this assay requires prolonged patient preparation (approximately 1 hour) and careful sample collection, which may be challenging if the collecting laboratory is unfamiliar with this test.
Stool Antigen Test
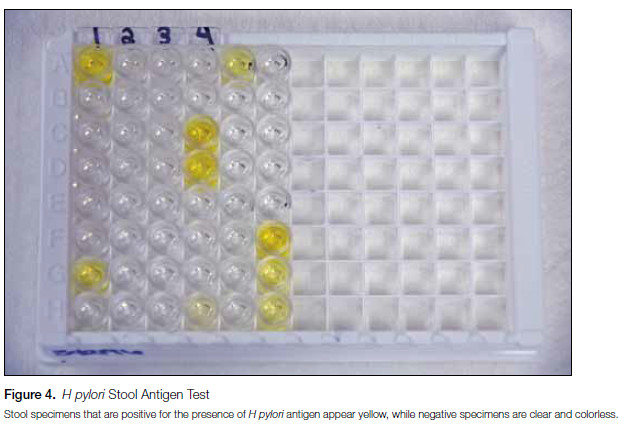
The stool antigen test (SAT) is an enzyme immunoassay (EIA) designed to detect H pylori in fecal specimens by measuring H pylori antigen released from organisms lining the stomach wall (Figure 4). As antigen is only detected if H pylori is present, the SAT can be used as an accurate tool to diagnose active H pylori infection and to establish eradication of the organism following treatment.3,11 However, similar to the UBT, current guidelines recommend performing confirmatory testing for clearance of infection at least 4 weeks following cessation of therapy since, in the interim, residual antigen may continue to be shed from nonviable organisms and lead to false-positive results. Though numerous SATs are commercially available, those using monoclonal antibodies to the H pylori antigen (including the assay used at Mayo Medical Laboratories), show high clinical sensitivity and specificity, typically greater than 90%.3,11 Unlike the UBT, specimen collection (ie, stool) is much simpler for SATs and this assay is approved by the US Food and Drug Administration (FDA) for use in pediatric patients.
Similar to the UBT, the sensitivity of the H pylori antigen test is decreased if the patient has taken PPIs, bismuth-containing compounds, or antibiotics up to 14 days prior to specimen collection. Therefore, if possible, these treatments should be withheld for at least 2 weeks prior to SAT testing. Additionally, while specimen collection is simpler compared to the UBT, patients may be uncomfortable with the process and may need to return to the clinic for specimen submission.
Serology
Currently, detection of antibodies (ie, IgM, IgA, IgG) to H pylori remains the most commonly ordered test by most providers to diagnose H pylori infection, due to the ease of specimen collection and single time-point testing. Additionally, unlike other noninvasive methods (ie, UBT and SAT), serologic profiles are not affected by prior use of PPIs, bismuth-containing compounds, or antibiotics, making serology an increasingly attractive method for immediate patient evaluation.
Despite these advantages, serologic testing for antibodies to H pylori has very limited clinical utility, outside of a general epidemiologic role. The greatest concern regarding this testing method is the poor positive predictive value (PPV) for many of these assays, particularly in regions of low H pylori endemicity (20%-30%), which represents the majority of the United States.3,12 Therefore, while a negative serologic result suggests the absence of prior exposure to H pylori, a positive result cannot be used to predict the presence of active disease. Importantly, the performance characteristics of these assays are poor, with clinical sensitivity and specificity parameters ranging from 76% to 85% and 79% to 90%, respectively, compared to culture and/or histopathology.3,13 Additionally, as antibodies to H pylori remain detectable for years following resolution of infection, serologic testing cannot be used to distinguish active from past infection or to document eradication of the organism following successful treatment. From a regulatory standpoint, while the UBT, SAT, and some H pylori IgG assays are FDA approved for use in most patient populations, there are currently no FDA-approved assays to detect H pylori IgM or IgA. Finally, an increasing number of health insurance providers, including Cigna, Geisinger Health, Aetna, and Blue Cross Blue Shield, are no longer reimbursing patients for whom H pylori serologic testing was performed, as they consider such testing “not medically necessary.”
Based on these many limitations and recent changes to patient reimbursement policies, Mayo Medical Laboratories will no longer offer serologic testing for antibodies to H pylori. As an alternative, providers are encouraged to amend their testing strategies by replacing serologic testing with either the UBT or the SAT assay for evaluation of patients with suspected H pylori infection. Both the UBT and SAT are offered through Mayo Medical Laboratories.
The Experience at Mayo Clinic
To conform to current H pylori testing guidelines and to improve patient management, leadership from Mayo Clinic’s divisions of Clinical Microbiology and Gastroenterology worked together to update our algorithm to detect H pylori infection. As a result, Mayo Medical Laboratories will discontinue all H pylori serologic testing in February 2016. Mayo Clinic providers will have the option of ordering either the SAT or the UBT for patients with suspected H pylori infection. Serologic evaluation of patients for H pylori is no longer considered clinically useful and as many insurance companies are no longer covering patient cost for such testing, these assays will no longer be offered through Mayo Medical Laboratories.
The most up-to-date testing algorithm for H pylori can be found on the Mayo Medical Laboratories website (www.mayomedicallaboratories.com) and is provided as an insert in this issue.

Summary
In conclusion, this article outlines the advantages and limitations of the available invasive and noninvasive testing methods to diagnose H pylori infection. By working collaboratively with our Mayo Clinic gastroenterology colleagues, the Clinical Microbiology Laboratory has successfully improved ordering practices, replacing serologic testing with either the SAT or the UBT to evaluate patients with suspected H pylori infection. Implementation of this practice change will lead to more accurate diagnosis of active H pylori infection and is expected to decrease the number of prescriptions for unnecessary antimicrobial agents.
Authored by Elitza S Theel, PhD